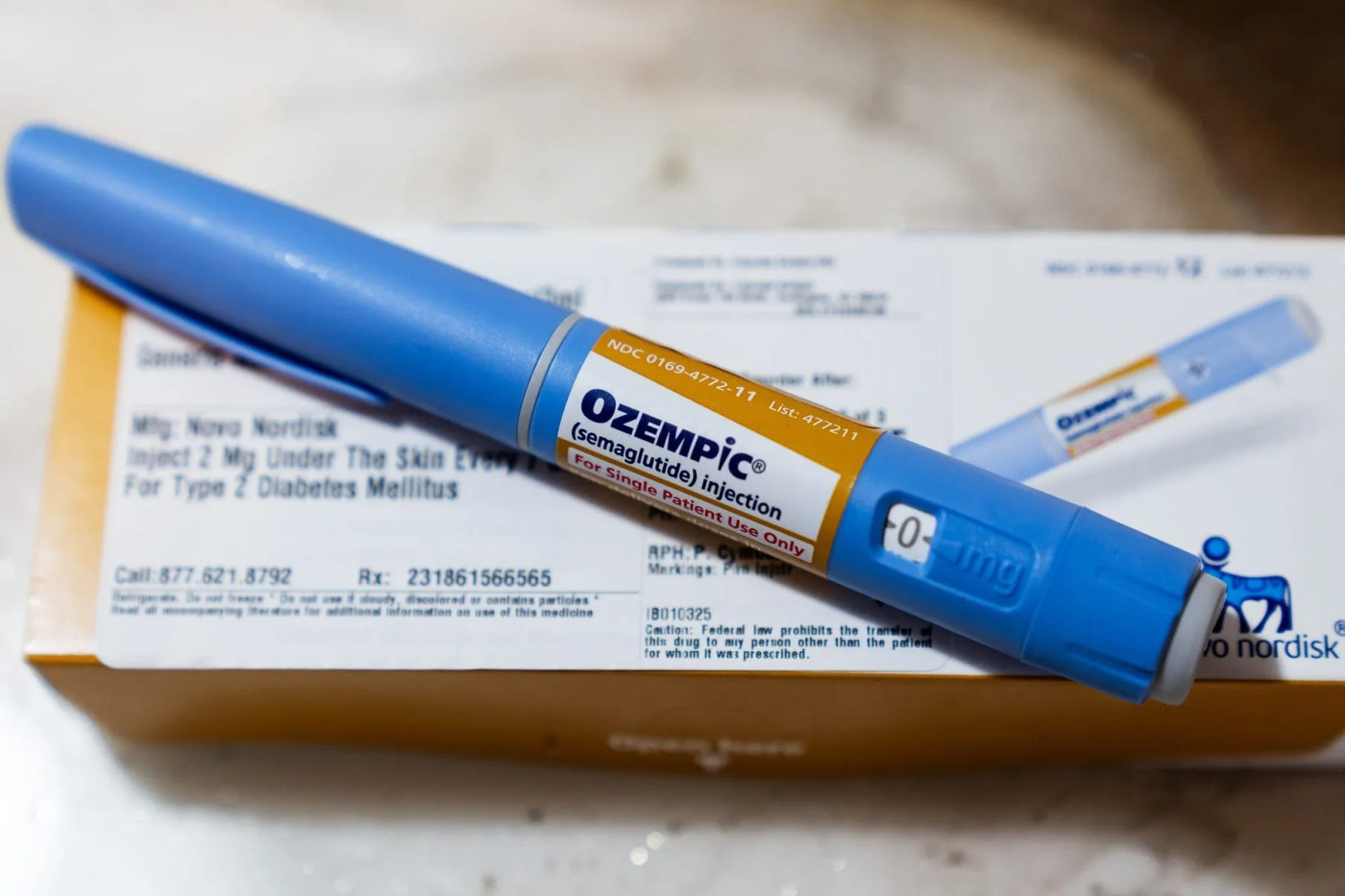
Shortage of popular drugs Wegovy and Ozempic is over, FDA says.
The Food and Drug Administration on Friday said the shortage of Novo Nordisk's weight-loss and diabetes drugs Wegovy and Ozempic is resolved, a move that could limit the availability of cheaper compounded versions of the wildly popular medications.
In a statement, the FDA said it confirmed Novo Nordisk's "stated product availability and manufacturing capacity" of these prescription drugs meets or exceeds current and projected demand. The agency warned consumers and doctors might still experience "limited localized supply disruptions" as the products are shipped from drug factories to distributors to pharmacies.
The Danish drugmaker Novo Nordisk said it has invested $6.5 billion this year in the United States to bolster drug production facilities that are operating 24/7, shipping Wegovy and Ozempic to wholesalers.
Pharmaceutical giants that market lucrative weight-loss drugs have battled with firms that sell less expensive versions made by compounding pharmacies.
The FDA allows compounding pharmacies to sell copies of drugs when the medications are in short supply. But compounding pharmacies face stiffer restrictions when a drug shortage is resolved.
Need a break? Play the USA TODAY Daily Crossword Puzzle.
Companies that market compounded drugs − combining, mixing or altering drug ingredients − have prospered amid shortages of the class of weight-loss medications, called GLP-1 (glucagon-like peptide-1) receptor agonists.
Hims & Hers, which launched a Super Bowl ad touting its weight loss product offerings, markets a compounded version of semaglutide, the active ingredient in Ozempic and Wegovy. Shares of Hims & Hers plunged 17% in mid-morning trading after the FDA's announcement.
The FDA said it won't take enforcement action against pharmacies or doctors that make compounded semaglutide due to the shortage before April 22. Facilities that compound, distribute or dispense semaglutide injections won't face enforcement action before May 22.
In December, the FDA declared that Eli Lilly's weight loss and diabetes medication tirzepatide, sold under the brand names Mounjaro and Zepbound, was no longer in short supply. That meant pharmacies had to discontinue "compounding, distributing or dispensing" tirzepatide as of Feb 18. Suppliers that produce batches of the drug and sell to others have until March 19 to cease distribution.
Compounding pharmacies are regulated by state boards of pharmacy and source ingredients are usually obtained from factories registered with the FDA. However, the federal agency doesn't verify the safety or effectiveness of compounding pharmacies.

